ChemInform Abstract: Studies Towards the Preparation of Sparteine-Like Diamines for Asymmetric Synthesis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
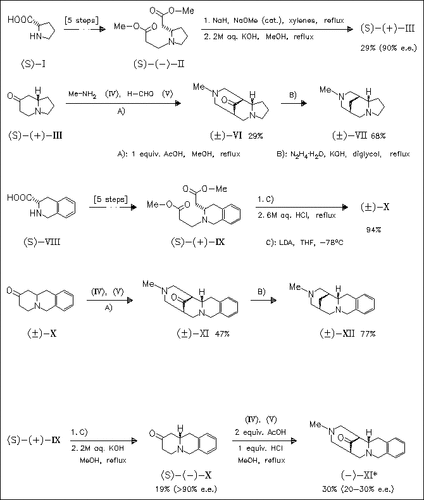
Two novel title derivatives (VII) and (XII) are prepared in racemic form starting from (S)-proline (I) or (S)-phenylalanine derivative (VIII). The routes involve Dieckmann condensation followed by double Mannich reaction to set up the tricyclic structure with control of the relative stereochemistry. During the Dieckmann and Mannich reactions racemization occurs. Conditions for preventing racemization in the Dieckmann condensation are found, but under different conditions the Mannich reactions always proceed with racemization.