ChemInform Abstract: Synthesis of Carbasugars from Aldonolactones. Part 2. Preparation of Polyhydroxy/aminocyclopentanes Functionalized at All Five Ring Carbons.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
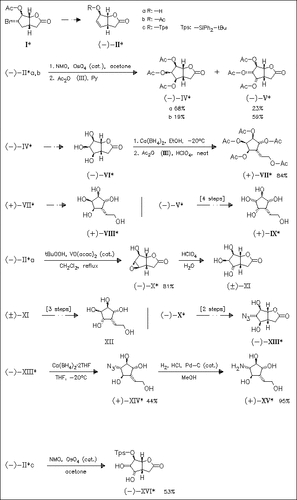
Four sugar mimics (VIII), (IX), (XII), and (XV) are prepared from the easily available bromo acetate (I). It is found that the stereoselectivity of the OsO4-catalyzed dihydroxylation step of allylic derivatives (II) depends on the size of the substituent at the hydroxy group and gives either predominantly or exclusively exo or endo products (IV), (V), or (XVI). Regioselective ring opening of lactones derived from (IV) or (V) is achieved either with in situ generated Ca(BH4)2 to give, after deprotection, the target compounds (VIII), (IX), and (XII) or with Ca(BH4)2×2THF complex to afford finally mimic (XV).