ChemInform Abstract: Syntheses of Methyl (4,6-Dideoxy-α-L-lyxo-hexopyranosyl)-(1→3)- and (4-Deoxy-4-fluoro-α-L-rhamnopyranosyl)- (1→3)-2-acetamido-2-deoxy-α-D-glucopyranosides, Analogues of the Mycobacterial Arabinogalactan Linkage Disaccharide.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
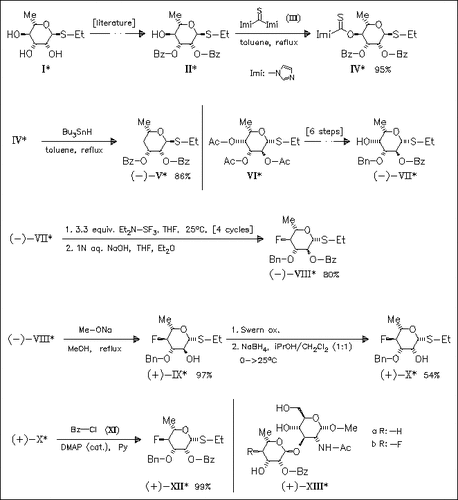
4-Deoxy-L-rhamnosyl and 4-fluoro-4-deoxy-L-rhamnosyl donors (V) and (XII) are prepared for the synthesis of disaccharides (XIII) which are prototypes for a family of potential inhibitors of the enzymes involved in the early stages of cell-wall construction in mycobacteria. The preparation of donor (XII) reveals difficulties in the fluorination step using diethylaminosulfur trifluoride. The desired fluoride (VIII) can be obtained in 80% yield by repeating the two-step sequence (fluorination and quenching) for four times maintaining the concentration of unreacted substrate and of reagent essentially constant at each cycle. The required inversion of the stereochemistry of the 2-position of fluoride (IX) is accomplished by an oxidation/reduction sequence.