ChemInform Abstract: Application of Sugar Phosphonates for the Preparation of Higher Carbon Monosaccharides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
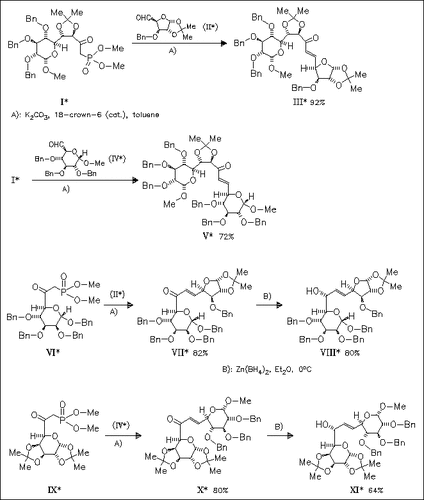
Sugar phosphonates as (I), (VI),and (IX), which can be prepared in much higher yields and which are much more nucleophilic than previously used sugar phosphoranes, react with sugar aldehydes, e.g. (II) and (IV), to generate the appropriate enones with trans configuration of the double bond. Whereas reduction of enone (III) is accomplished in low selectivity, affording an inseparable mixture of sugar allylic alcohols, reduction of enones (VII) and (X), which have the carbonyl group placed at the α-position of the sugar ring, yields allylic alcohols (VIII) and (XI) in high stereoselectivity.