ChemInform Abstract: Conversion of D-Xylose to Protected D-Lyxose Derivatives and to D-Lyxose, via the Corresponding 1,2-Anhydride.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
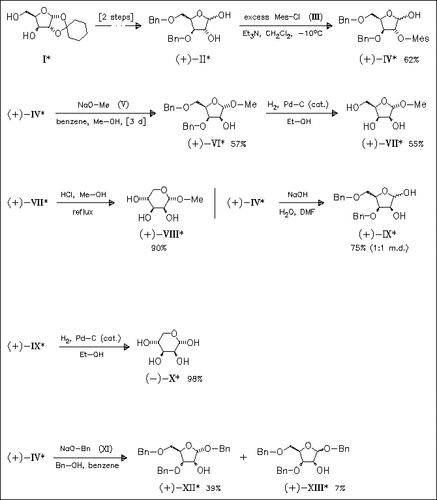
Certain lyxofuranosides are prepared from suitably protected xylose derivatives (IV) using similar conditions as for the preparation of methyl β-D-ribopyranoside. Xylofuranoside (IV) reacts readily with sodium salts to give the protected lyxofuranosides. A possible mechanism presumably involves initial formation of the corresponding 1,2-anhydro derivative as an intermediate. Finally, these compounds are converted into lyxoses (VIII) and (X) or into α-lyxopyranoside (XII). The sequence (IV)→(IX)→(X) affords a novel pathway from xylose to lyxose.