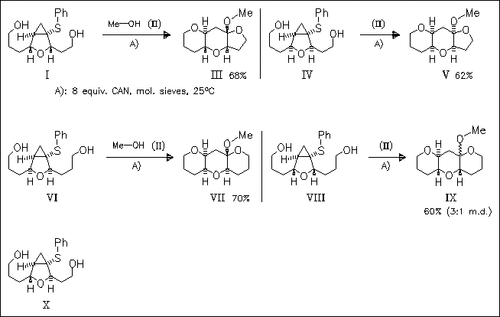
ChemInform Abstract: CAN Induced Double Ether Ring Formation: Synthesis of trans- and cis-Fused Tricyclic Ethers from 3-Oxabicyclo[3.1.0]hexyl Sulfides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Oxidation of title oxabicyclohexyl sulfides (I), (IV), (VI) and (VIII) containing two hydroxy substituents in the C-2 and C-4 side chains proceeds by double ether ring formation and concurrent cleavage of the most substituted cyclopropyl bond to afford tricyclic ketals (III), (V), (VII) and (IX), respectively. In most cases, this tandem cyclization reaction proceeds with absolute diastereoselectivity. Tricyclic ketals are transformed to the corresponding tricyclic ethers by TmsOTf-promoted reduction in the presence of Et3SiH. The trans-substituted oxabicyclohexyl sulfide (X) does not afford any bicyclized product, which demonstrates that a sterically defined alignment of nucleophile and fissile cyclopropyl bond is necessary to achieve double ether cyclization.