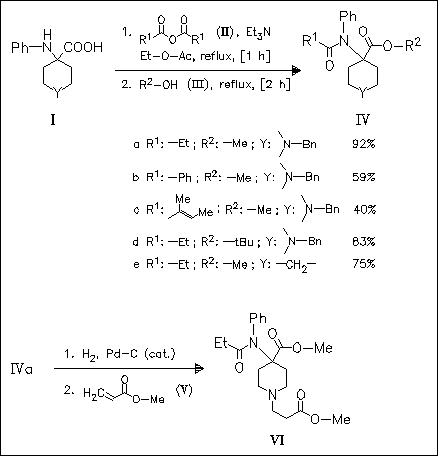ChemInform Abstract: A Convenient Method for the N-Acylation and Esterification of Hindered Amino Acids: Synthesis of Ultra Short Acting Opioid Agonist, Remifentanil.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
An easily performed sequence allows the conversion of α-amino acids of type (I) to N-acylamino acid esters (IV), isolated as oxalates. Derivative (IVa) is a valuable precursor of remifentanil (VII).