ChemInform Abstract: Asymmetric Synthesis of Cyclopropane-1,1-dicarboxylates from a γ-Alkoxy-alkylidenemalonate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
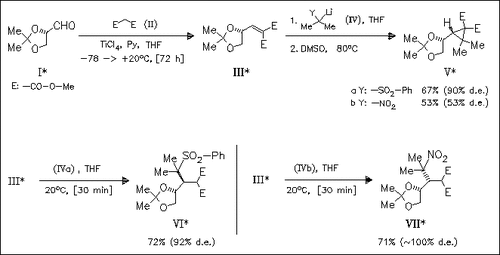
Cyclopropanation of the D-mannitol derived alkylidenemalonate (III) with 2-lithio-2-sulfonyl- and 2-lithio-2-nitropropane (IV) provides the same diastereomer (V) from both reagents, however with different diastereoselectivity. On the other hand, formation of the open chain derivatives (VI) and (VII) occurs with almost completely different face-selectivity. Thus, whereas open chain derivative and the cyclopropane arise from the same face of (III) from (IVa), they results from a different face of attack for (IVb). The reason for this discrepancies are unclear.