ChemInform Abstract: Development of a Chiral Propionaldehyde Homoenolate Equivalent Which Reacts with Imines with Excellent Stereoselectivity: Efficient and Practical Synthesis of Optically Active γ-Amino Carbonyl Compounds.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
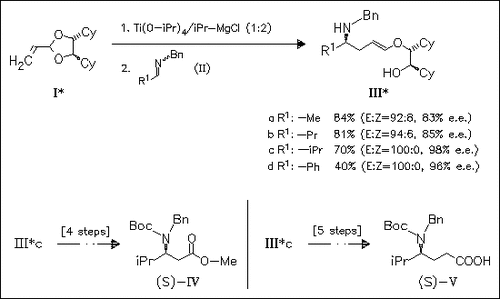
An allyltitanium intermediate formed by reaction of acetal (I) with (η2-propene)Ti(O-iPr)2 reacts with imines such as (II) to give optically active α-addition products (III) predominantly as (E)-enol ethers. Pure (E)-isomers with 96—98% e.e. are isolated from the E/Z product mixtures of (IIIc) and (IIId). The versatility of the enol ether functionality is demonstrated by conversion of derivative (IIIc) into β-amino ester (IV) and γ-amino acid (V).