ChemInform Abstract: (-)-Sparteine-Mediated Asymmetric Cyclocarbolithiation of Alkenes Combined with a Stereospecific retro-[1,4]-Brook Rearrangement.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
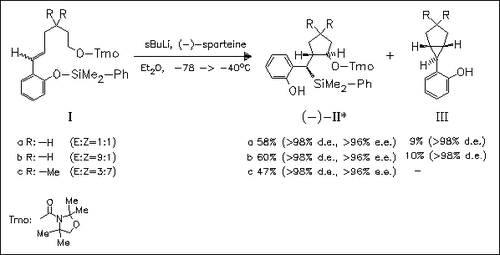
A novel stereoselective cyclocarbolithiation—retro-[1,4]-Brook rearrangement reaction cascade of silyloxyarylhexenyl carbamates (I) is described. The internal [O→C] silyl transfer proceeds with retention of the configuration at the benzylic position to furnish optically pure (α-silylbenzyl)cyclopentane derivatives (II), and, moreover, it suppresses the undesired 1,3-cycloelimination to bicyclic compounds (III).