ChemInform Abstract: Asymmetric Synthesis of 4′-Methyl-2′,3′-dideoxynucleosides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
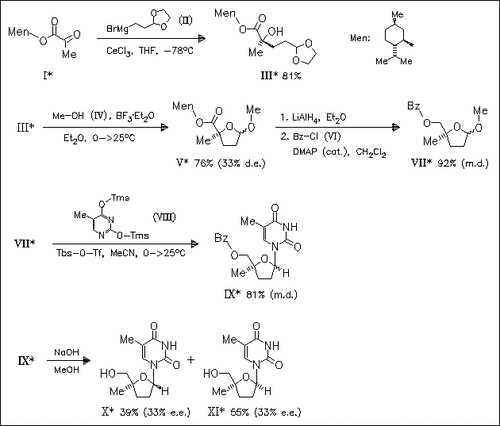
Asymmetric stereoselective preparation of a 4-methyl-2,3-dideoxyribose derivative is described which utilizes menthyl pyruvate as a chiral template and an organocerium addition as the key carbon—carbon bond-forming step. This compound (IX) is used as a substrate for a Vorbrueggen pyrimidine glycosylation giving an anomeric mixture of title compounds (X) and (XI).