ChemInform Abstract: Enantioselective Highly Efficient Synthesis of (-)-Cephalotaxine Using Two Palladium-Catalyzed Transformations.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
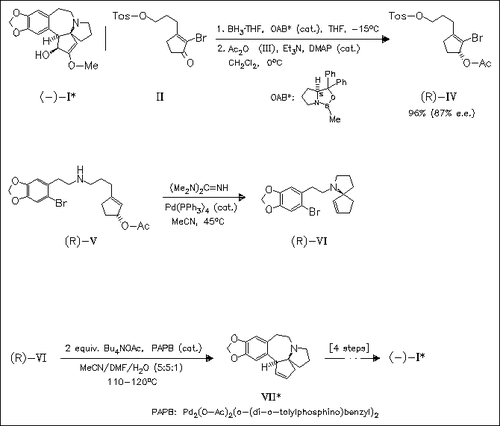
The highly efficient synthesis of title compound (I) involves enantioselective reduction of the brominated cyclopentenone derivative (II) for introduction of chirality and two successive Pd-catalyzed reactions as key reactions, i.e. allylation of sec. amine (V) with complete retention and diastereoselective Heck reaction of spirocyclic amine (VI).