ChemInform Abstract: Synthesis of Enantiomerically Pure β- and γ-Amino Acid Derivatives Using Functionalized Organozinc Reagents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
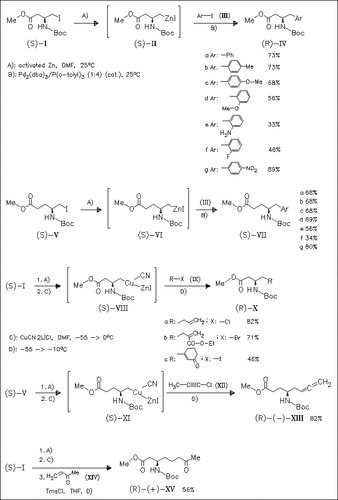
New functionalized organozinc reagents (II) and (VI) are prepared from amino acid derivatives (I) and (V) by treatment with activated zinc dust. The use of dipolar aprotic solvents is essential to minimize β-elimination of the carbamate group. Furthermore, the use of such solvents allows the convenient and reliable formation of such zinc reagents under mild conditions and successful cross-coupling reactions with aryl iodides to afford the title amino acid derivatives. The related zinc/copper reagents (VIII) and (XI) are also useful intermediates for cross-coupling reactions with electrophiles.