ChemInform Abstract: Conformationally Restricted Paclitaxel Analogues: Macrocyclic Mimics of the “Hydrophobic Collapse” Conformation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
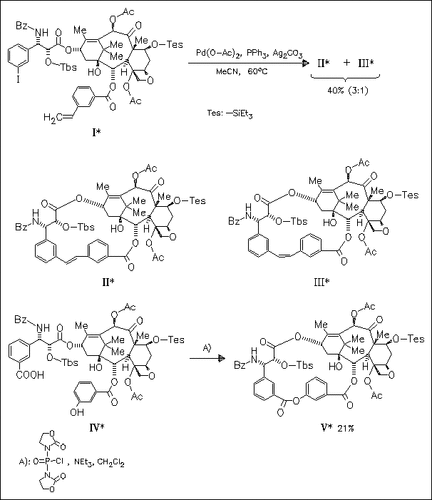
The synthesis of conformationally restricted macrocyclic taxol analogues designed to mimic the hydrophobically collapsed conformation is described. Key steps of the syntheses are an intramolecular Heck reaction and a macrolactonization under high dilution conditions, respectively. NMR analysis shows that compound (V) is a mixture of two atropisomers. The analogues do not show activity in a tubulin assembly assay.