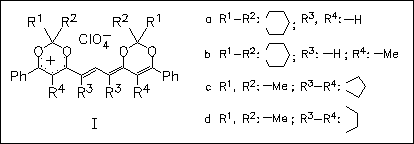ChemInform Abstract: Acetals and Vinyl Ethers of Unsaturated Aldehydes and Ketones in New Syntheses of Heterocyclic Compounds. Part 10. Configurational Z,E Isomerism of 1,3-Bis(6-phenyl-1,3-dioxin-4-ylidene)propan-2-ylium Perchlorates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Trimethinecyanines (I) are synthesized from dioxinylium salts using the conventional procedure for methynecyanines. The title salts (I) exist as three configurational Z,E isomers which undergo fast and reversible interconversions at elevated temperature. Quantum-chemical calculations reveal two symmetric and one asymmetric configurations of the carbocation. Terminal carbon atoms of the trimethine bridge which are incorporated into five- or six-membered rings exhibit no Z,E isomerism of trimethinecyanines.