ChemInform Abstract: Preparation and Cyclopropanation of Selected Oxapolycycles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
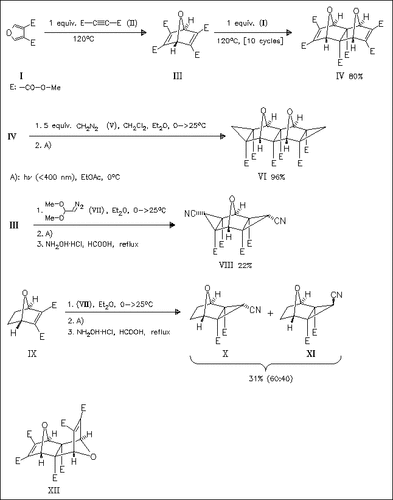
Diels—Alder cycloaddition of furandicarboxylate (I) with equimolar amounts of DMAD (II) affords oxabicycloheptadiene (III), which undergoes a further cycloaddition with excess furandicarboxylate (I) to afford a mixture of exo,exo-bisadduct (IV) and exo,endo-bisadduct (XII) in circa 20% yield each. Crystallization and repeated cycloaddition of the mother liquor, however, allows the enrichment of bisadduct (IV). Cyclopropanation of compound (IV) with diazomethane proceeds at both double bonds to afford dioxahexacyclotetradecane derivative (VI). In contrast, cyclopropanation with a “latently acceptor-substituted” diazomethane (VII) obviously proceeds with retro Diels—Alder reaction to give compound (III), which is subsequently cyclopropanated. Interestingly, only one of three possible diastereomers is obtained, while the analogous cyclopropanation of oxabicycloheptene (IX) affords a mixture of diastereomers (X)/(XI).