ChemInform Abstract: Catalytic Enantioselective Conjugate Addition of 1,3-Dicarbonyl Compounds to Nitroalkenes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
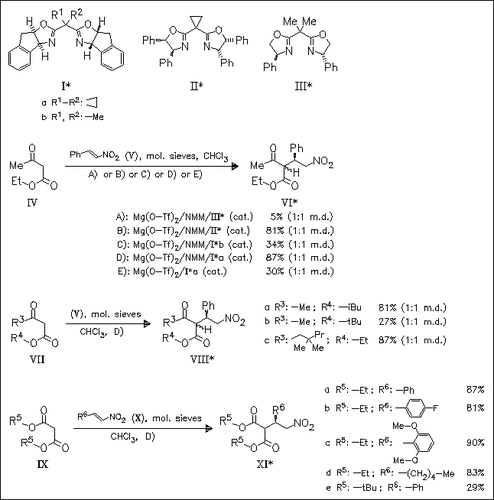
The first highly enantioselective catalytic conjugate addition of β-ketoesters (IV) and (VII) as well as malonates (IX) to nitroolefins (V) or (X) is presented. The reaction proceeds smoothly under catalysis of Mg(O-Tf)2×4H2O—bis(oxazoline) (Ia) in the presence of N-methylmorpholine (NMM) as cocatalyst. Optimization of reaction conditions, concerning solvent, presence of water and NMM, and nature of chiral ligand, is presented. The reaction is shown to proceed with a wide variety of substituted nitrostyrenes as well as alkyl-substituted nitroolefins. Additionally, steric bulk of the ester affects rate and selectivity of the addition reaction.