ChemInform Abstract: Preparation of Ketones from Esters via Substituted Cyclopropanols. Application to the Synthesis of (.+-.)-Ipsenol, (.+-.)-Ipsdienol and Amitinol, the Components of Aggregation Pheromones of the Ips Bark Beetles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
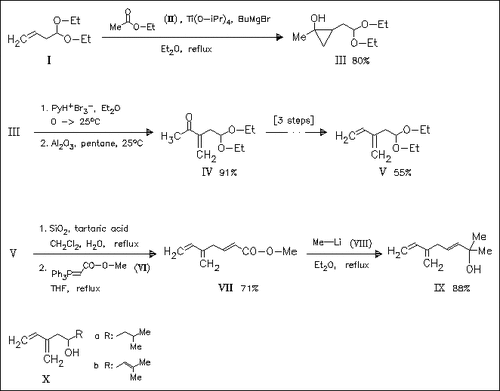
Key step in the synthesis of title pheromones (.+-.)-ipsenol (Xa), (.+-.)-ipsdienol (Xb), and amitinol (IX) is the preparation of methyl ketone (IV) starting from an ester. Titanium-promoted addition of ethyl acetate to the double bond of vinylacetic aldehyde acetal (I) provides a cyclopropanol derivative (III), which is regioselectively opened by a bromination—dehydrobromination sequence. Reduction of the ketone, chlorination, and dehydrochlorination reactions then afford diene acetal (V) as key intermediate, which provides the title pheromones by reaction with the suitable Grignard reagents or by Wittig olefination.