ChemInform Abstract: Efficient Route to Functionalized Eight-Membered Lactones Based on Intramolecular Silicon-Directed Acylative Ring-Opening Reactions of 3-(Tetrahydro-2-furyl)propanoic Acid Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
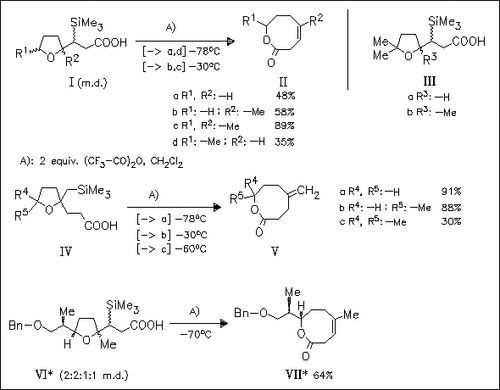
The ring enlargement of furylpropanoic acids (I), (IV), and (VI) by treatment with trifluoroacetic anhydride is an effective method for the synthesis of eight-membered lactones. In some cases, products of other ring-rearrangements are isolated additionally in low yields [from compound (Id) and (IVc)] or exclusively [from compounds (III)].