ChemInform Abstract: Synthesis of Isoindolo[2,1-a]indoles by the Palladium-Catalyzed Annulation of Internal Alkynes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
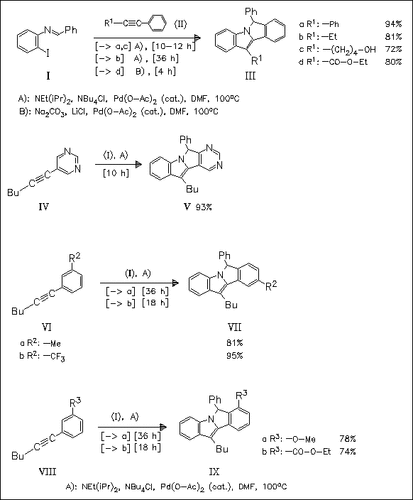
A remarkable Pd-catalyzed annulation process forming the title compounds from readily available imine (I) and aryl acetylenes is presented. Following mechanism is proposed: 1.) generation of the active Pd(0) catalyst from Pd(O-Ac)2, 2.) oxidative addition of aryl iodide (I) to Pd(0), 3.) coordination and subsequent insertion of the acetylene, 4.) 5-exo addition of the intermediate vinylpalladium species to the C=N bond, 5.) either electrophilic palladation of the resulting σ-palladium species to the adjacent aromatic ring or oxidative addition of the neighboring aryl C—H bond to the σ-palladium species generating a Pd(IV) intermediate, and subsequent elimination of HI, 6.) reductive elimination forming the isoindole. This reaction provides the first example of a vinyl palladium species adding to a C=N bond. The regiochemical switch observed in the reactions of acetylenes (VI) and (VIII) is explained by a chelation effect.