ChemInform Abstract: Ruthenium-Catalyzed ortho Vinylation of Aromatic Ketones with Alkynes: Unexpected Cyclopentannulation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
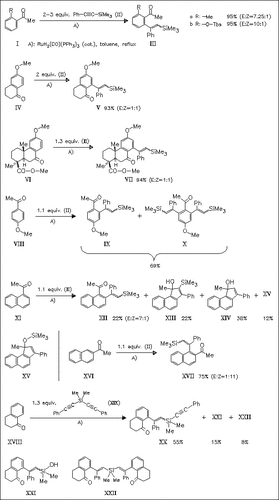
Various aromatic ketones give under treatment with alkynylsilanes in most cases ortho-vinylated products. The predominant geometry of the newly introduced double bond is E in all but a few cases. In contrast, 1-acetylnaphthalene undergoes a one-pot insertion sequence yielding cyclopenta[a]naphthalene derivatives. The reaction of bisacetylene (XIX) with 1-tetralone (XVIII) affords a product mixture containing diarylated silane (XXII) as single stereoisomer.