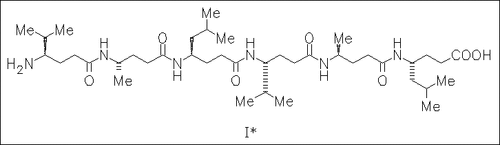
ChemInform Abstract: γ-Peptides Forming More Stable Secondary Structures than α-Peptides: Synthesis and Helical NMR-Solution Structure of the γ-Hexapeptide Analogue of H-(Val-Ala-Leu)2-OH.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The title γ-hexapeptide (I) is synthesized by double Arndt—Eistert homologation of the corresponding protected amino acids and conventional peptide-coupling method. NMR measurements reveal that (I) adopts a right-handed helical structure helix.