ChemInform Abstract: Ti(II)-Mediated Allenyne Cyclization as a New Tool for Generation of Chiral Organotitanium Compounds. Asymmetric Generation of Cyclic Allyltitanium Reagents with No Chiral Auxiliary.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
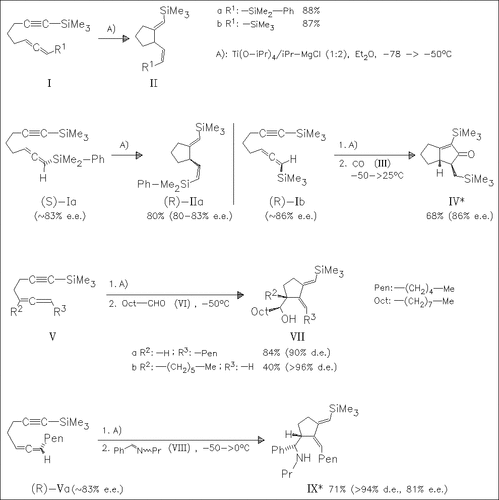
The cyclization of 1,2-dien-7-ynes, such as (I), and 1,2-dien-6-ynes, such as (V), with Ti complexes generated in situ from Ti(O-iPr)4 and iPr-MgCl leads to 1-alkenyl-2-alkylidenecyclopentanes in moderate to good yields. With chiral (V), the intermediate formation of new chiral titanabicycles allows the stereoselective addition to carbonyl compounds affording optically active substituted cyclopentanes, possible precursors of naturally occurring products.