ChemInform Abstract: Asymmetric Synthesis of Alkannin and Shikonin.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
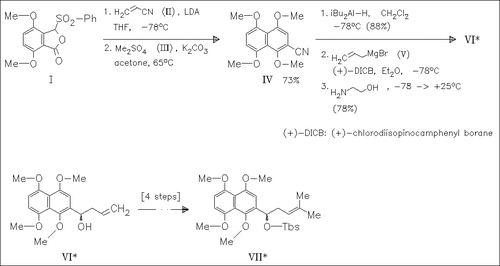
A new route for the synthesis of the fully protected shikonin (VII) is described. The procedure is based on the condensation of an 1,4-dipole equivalent with the Michael acceptor acrylonitrile (II) to construct the polyoxygenated aromatic ring system and the introduction of the chiral center via an asymmetric allyl boration. Using the antipode of the allyl boration reagent leads to the synthesis of (-)-alkannin.