ChemInform Abstract: Stereoselective Synthesis of the C3—C17 Bis-oxane Domain of Phorboxazole A.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
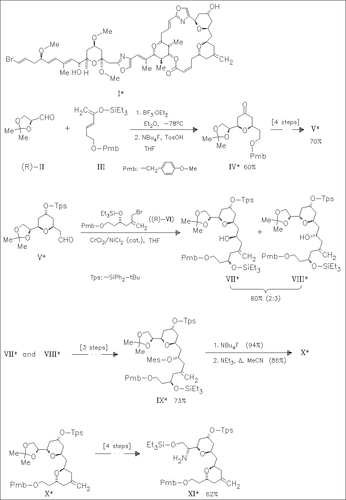
The naturally occurring title compound phorboxazole A (I) has an exceptionally potent cytostatic activity against a wide range of human tumor cell lines. The central core (C(18)—C(30)) has been reported. This work describes the synthesis of the complementary C(3)—C(17) portion (XI) of the macrolide, involving sequential assembly of both the oxanes by hetero Diels—Alder and intramolecular etherification processes, respectively.