ChemInform Abstract: A New Photoannulation Reaction of 2-Aryl-3-alkoxy-1,4-naphthoquinones. Synthesis of Dimethylnaphthgeranine E.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
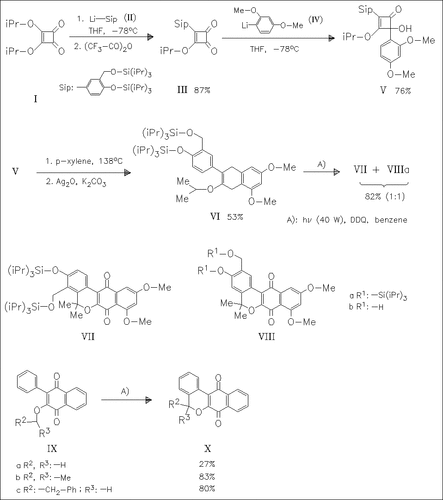
For the first time, a direct analogue of the naphthgeranine family of natural products, the title compound (VIIIb), is synthesized. Key steps in the synthesis are the thermally induced ring expansion of 4-arylcyclobutenones for the regiospecific synthesis of the naphthoquinone (VI), and its new and facile photoannulation reaction for the construction of the pyranonaphthoquinone nucleus in (VIIIa) and its regioisomer (VII). Desilylation of (VIIIa) gives the title compound (VIIIb) with 76% yield. The scope of the photoannulation was further probed and found to have useful generality. The synthetic strategy employed here will be applicable to the synthesis of other natural quinones.