ChemInform Abstract: Phosphonate Diester and Phosphonamide Synthesis. Reaction Coordinate Analysis by 31P NMR Spectroscopy: Identification of Pyrophosphonate Anhydrides and Highly Reactive Phosphonylammonium Salts.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
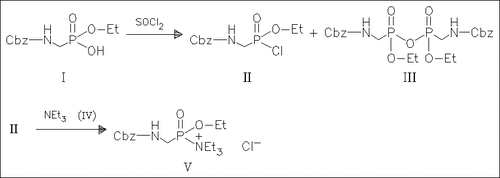
Pyrophosphonate anhydrides are formed as by-products in the preparation of phosphonochloridates from phosphonate monoesters with thionylchloride. The mechanism is discussed and the reactions with alcohols and amines are investigated by 31P NMR spectroscopy. The anhydrides are less reactive toward nucleophiles, particularly toward amines than the corresponding phosphonochloridates. Importantly, treatment of the phosphonochloridates with tert. amines prior to the addition of nucleophile results in the formation of phosphonylammonium salts, which are highly reactive phosphonylating agents superior to phosphonochloridates, affording higher yields of phosphonate esters and amides.