ChemInform Abstract: 1,3-Dipolar Cycloaddition Reactions of Nitrones with Alkyl Vinyl Ethers Catalyzed by Chiral Oxazaborolidines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
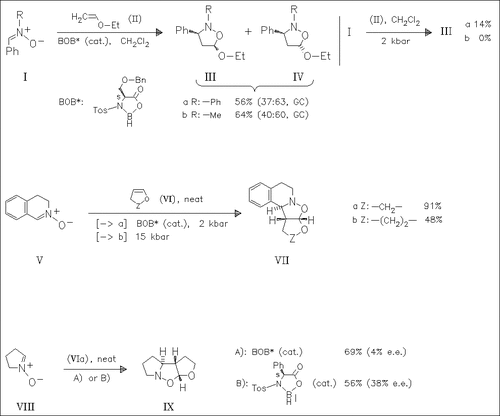
It is found that 1,3-dipolar cycloadditions of various nitrones with ethyl vinyl ether are catalyzed by chiral oxazaborolidines. The reaction proceeds with complete regioselectivity but with poor stereoselectivity and very low enantioselectivity (ca. 0% e.e.). With rigid (Z)-alkyl vinyl ether drastically improved stereoselectivity is observed. Starting from the nitrone (VIII) a cycloadduct with moderate enantioselectivity can be obtained.