ChemInform Abstract: 2-Functionalized Furans as Precursors of Versatile Cycloheptane Synthons.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
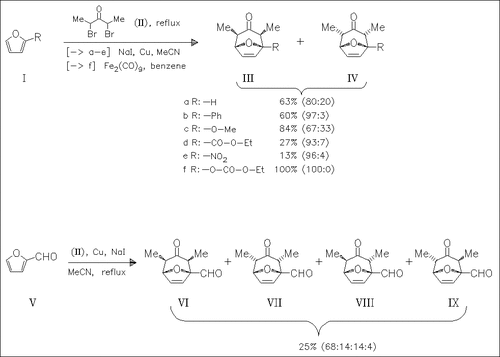
The outcome of the [4 + 3]cycloaddition, which in most cases proceeds with cis-diastereospecificity and high endo selectivity, is found to depend on steric and electronic effects. Increasing bulkiness of the 2-substituents results in increase of endo selectivity, but decrease of yield. Increasing electronic density of the furan system by electron donating groups at C-2 leads to better yields and diastereoselectivities.