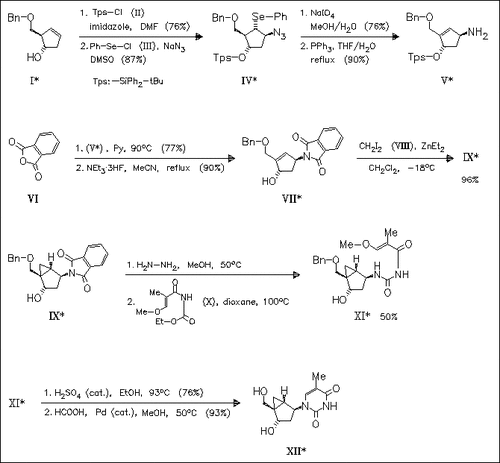
ChemInform Abstract: (1S,2R)-((Benzyloxy)methyl)cyclopent-3-enol. A Versatile Synthon for the Preparation of 4′,1′a-Methano- and 1′,1′a-Methanocarbocyclic Nucleosides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The key steps in the synthesis of the 4′,1′a-methanocarbocyclic nucleoside (XII) from the title enol (I) are regio- and stereoselective azidoselenylation of the olefin (I) and Simons-Smith cyclopropanation of the olefin (VII). Formation of a 1′,1′a- methanocarbocyclic nucleoside from (I) is previously reported.