ChemInform Abstract: Direct Glycosylations with 1-Hydroxy Glycosyl Donors Using Trifluoromethanesulfonic Anhydride and Diphenyl Sulfoxide.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
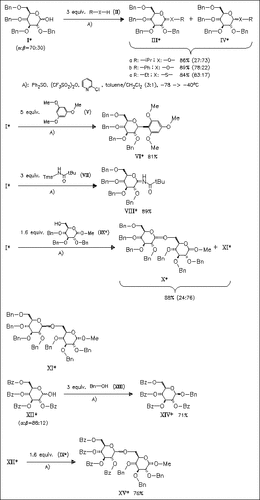
A new method for glycosidic bond construction is described involving in situ activation of the anomeric hydroxyl. This one-step procedure is applicable directly to the glycosylation of a wide range of acceptors. The C-2 neighboring group effects the glycosylation stereochemistry. In the case of (XII) β-selectivity is observed exclusively.