ChemInform Abstract: Pyrolytic Generation and Rearrangement of N-Substituted 1,2- Didehydrocarbazoles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
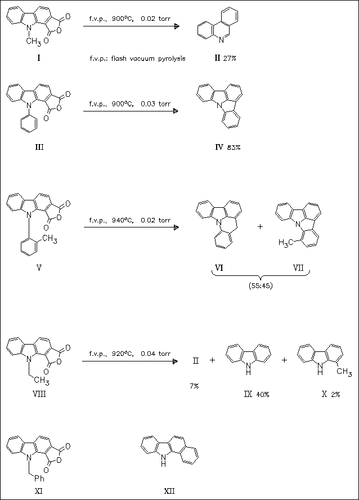
Flash vacuum pyrolysis of a series of anhydrides leads to intermediate 1,2-didehydrocarbazoles which mainly undergo ring expansion, dealkylation, and cyclization reactions. Ring contractions of the aryne system to exocyclic carbenes are not observed. The benzyl derivative (XI) gives a complex mixture in which products derived from initial N-Bn cleavage predominate (bibenzyl, benzaldehyde, benzyl alcohol, carbazole). The trace product (XII) is probably formed by an intramolecular cycloaddition.