ChemInform Abstract: Metallated 2-Alkenyl Sulfoximines in Asymmetric Synthesis: Regio- and Stereoselective Synthesis of Highly Substituted Tetrahydrofurans.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
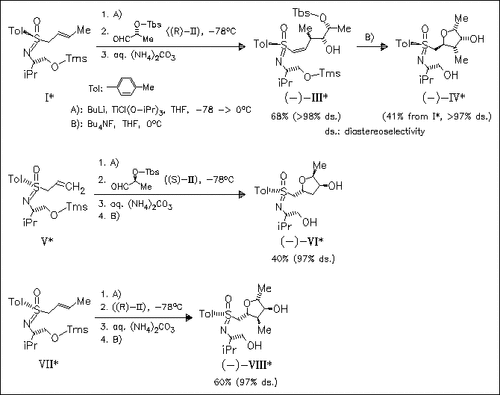
Starting from enantiopure sulfoximines (I), (V), (VII), and the epimer of (V), a one-pot procedure is developed for the synthesis of tri- and tetrasubstituted tetrahydrofurans. Intermediate Tbs-protected alcohols like (III), formed from the above sulfoximes and aldehydes (II), undergo fluoride-ion induced regioselective ring closure. The absolute configuration in the target furans is controlled at C-3 and C-4 by the sulfoxime moiety during aldehyde uptake, at C-2 by the proper choice of aldehyde (II), and at position 5 by the release of 1,3-allylic strain.