ChemInform Abstract: Palladium-Catalyzed Reactions. Part 1. Palladium-Catalyzed Enantioselective Hydrophenylation and Hydrohetarylation of Bicyclo(2.2. 1)hept-2-ene: Influence of the Chiral Ligand, the Leaving Group, and the Solvent.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
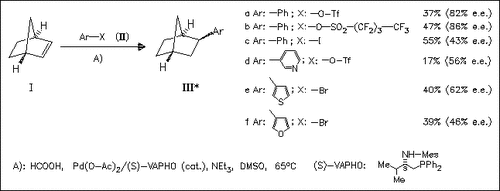
Various chiral P-P and P-N ligands (8 examples) are used in the Pd- catalyzed reaction of norbornene (I) with various phenyl and hetaryl derivatives (II). The best optical induction is achieved with the demonstrated valine-based P-N ligand. A systematic study is made of the influence of various hetaryl compounds, leaving groups, and solvent on the chemical and optical yields of this reductive arylation. Best enantioselectivities are achieved in DMSO with arylnonaflates forming products of type (III) with asymmetric induction of up to 86% e.e.