ChemInform Abstract: Palladium-Catalyzed Coupling of Optically Active Amines with Aryl Bromides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
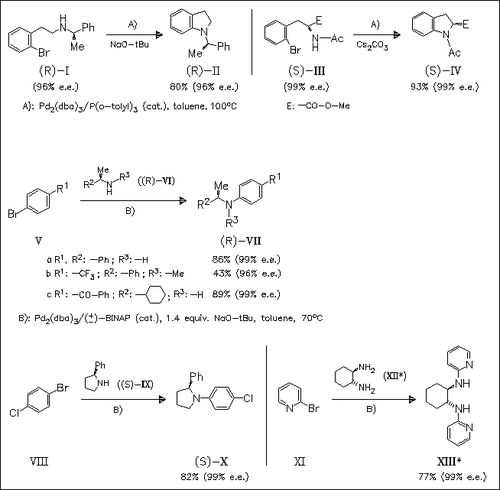
The choice of the ligand in the title reaction is critical to the formation of the anilines without loss of enantiomeric purity. The intramolecular coupling with the catalyst system in condition A) occurs without racemization whereas the intermolecular amination under these conditions leads to racemized products. However, the use of the catalyst system in condition B) preserves the optical purity of the starting amine. A mechanism for the observed racemization is proposed.