ChemInform Abstract: Intramolecular Cyclization Reaction via a Sterically Protected Episelenonium Ion Intermediate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
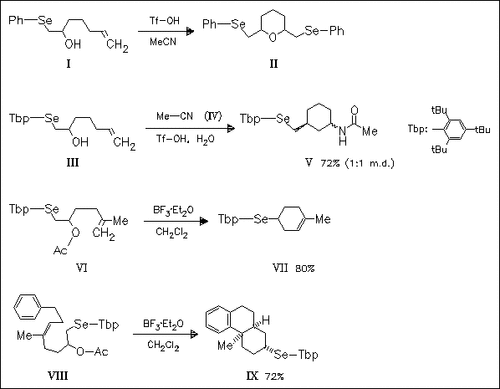
Steric protection of the selenium atom in the episelenonium ion intermediate by the bulky 2,4,6-tri-tert-butylphenyl group is utilized in the selective formation of carbocyclic compounds such as (V) and ( VII) from substrates (III) and (VI), respectively, bearing a β- oxyselenide unit and a double bond in the same molecule. In contrast, the olefinic alcohol (I), bearing the phenylseleno group, leads under the same conditions to the formation of the cyclic ether (II), indicating that the phenylseleno group migrates to the double bond of another molecule.