ChemInform Abstract: Tandem Inter (4 + 2)/Intra (3 + 2) Cycloadditions of Nitroalkenes. Part 15. The Bridged Mode (α-Tether).
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
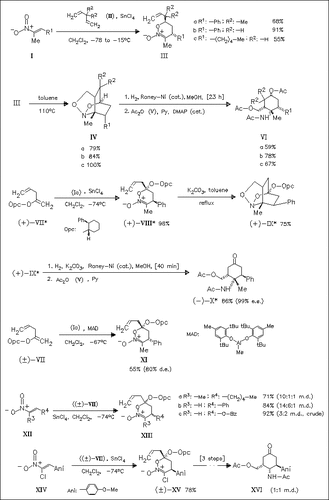
A new version of tandem inter (4 + 2)/intra (3 + 2) cycloaddition of nitroalkenes is reported. Simple 1,4-pentadienes and 2-alkoxy-1,4- pentadienes can function effectively as dienophile and dipolarophile combinations with excellent chemical selectivity and regio- and stereoselectivities. The stereochemical outcome depends on the α- substituent on the nitroalkene and the nature of Lewis acid used. Hydrogenation of the bridged nitroso acetals leads to aminocyclohexanemethanol derivatives with high diastereo- and enantioselectivity. A chlorine atom can be used successfully as hydrogen surrogate.