ChemInform Abstract: Chiral Lewis Acid Controlled Synthesis (CLAC Synthesis): Chiral Lewis Acids Influence the Reaction Course in Asymmetric Aldol Reactions for the Synthesis of Enantiomeric Dihydroxythioester Derivatives in the Presence of Chiral Diamines Derived from L-Proline.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
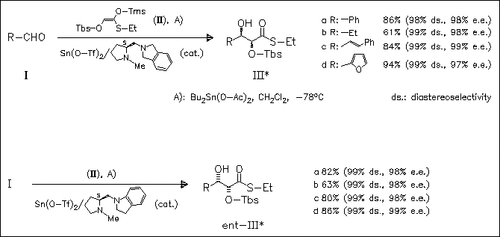
The preparation of both enantiomers of 2,3-dihydroxythioester derivatives with perfect selectivities by using similar types of chiral sources derived from L-proline is described. The chiral diamines used differ only in the fusion point of the benzene ring connected to the pyrrolidine moiety. The unique selectivities are ascribed to the conformational difference between the chiral tin(II) Lewis acids of the chiral diamines.