ChemInform Abstract: Synthetic Entry into the ent-Kaurene Framework. Application of an Unprecedented Transannular Cyclization for Forming the Central Bond Common to the B and C Rings.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
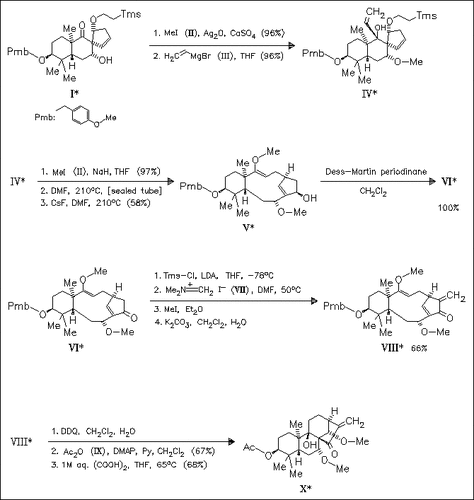
Starting with the known alcohol (I) the derivative (X) with the title framework is obtained. The key step in this approach is a transannular ring closure of the precursor (IV) with migration of a methoxy group leading to compound (V), whose structure is established by X-ray analysis.