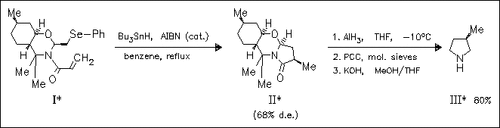
ChemInform Abstract: Diastereoselective 5-exo-trig Radical Cyclization on N-Acryloyl- tetrahydro-1,3-oxazines. A Novel Approach to Enantiopure 3-Substituted Pyrrolidines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Treatment of the phenylselenoylmethyl oxazine (I) with tributyltin hydride under radical conditions generates a carbon-centered radical which undergoes diastereoselective 5-exo-trig cyclization to the five- membered lactam (II), which is readily converted to enantiopure 3- methyl pyrrolidines (III).