ChemInform Abstract: Diastereoselective Synthesis of Highly Substituted 2,5-Diaminohexanes via Nitrile Oxide Cycloaddition to a Vinylogous Amino Acid.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
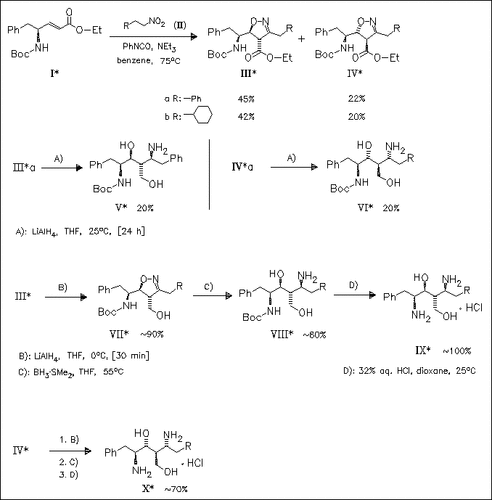
The 1,3-dipolar cycloaddition of the nitrile oxides derived from (II) to the pentenoate (I) gives a mixture of the syn and anti cycloadducts (III) and (IV). The individual isomers are converted to the desired 1, 4-diaminohexanes (required as inhibitors of HIV-1 protease) via selective cleavage of the isoxazoline ring.