ChemInform Abstract: Synthesis of 2-Azetidiniminium Salts. Part 1. Diastereoselectivity in Keteniminium Triflate/Imine Cycloadditions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
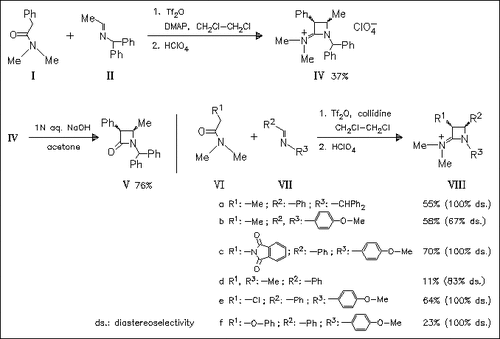
The iminium triflate salt/imine protocol for the title synthesis of cis-2-azetidinium salts (isolated as perchlorates), such as (IV) and ( VIII), is complementary to the one employd for the trans-specific chloride analogues and may constitute an alternative route to that of the Staudinger reaction for β-lactams, such as (V).