ChemInform Abstract: Application of Chemical P-450 Model Systems to Studies on Drug Metabolism. Part 10. Novel Hydroxylactonization of γ,δ- and β,γ-Unsaturated Carboxylic Acids with an Iron Porphyrin- Iodosylbenzene System.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
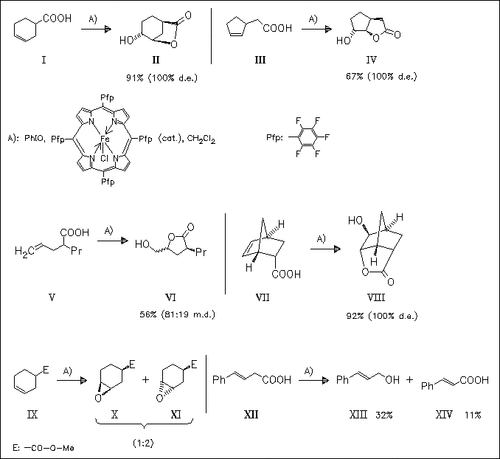
The title reaction is investigated with different kinds of unsaturated acids. The formation of hydroxy lactones is highly stereoselective. . beta.,γ-Unsaturated carboxylic acids are mainly decarboxylated. Lactone formation is observed in the case of iodomethacin, however with low yield and in a mixture of by-products. The lactones are also formed in the presence of microsomes. This metabolism is investigated and found to be cytochrome P-450 dependent.