ChemInform Abstract: Asymmetric Synthesis of δ-Keto Esters via Michael Additions of Chiral Carbene Complexes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
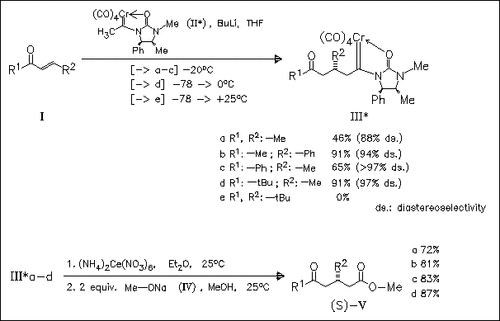
The asymmetric Michael addition of enolates of chiral imidazolidinone carbene complexes (II) to α,β-unsaturated ketones (I) occurs with high to excellent induction thus offering a convenient route to optically pure δ-keto esters (V).