ChemInform Abstract: Influence of the Sulfinyl Group on the Chemoselectivity and π- Facial Selectivity of Diels-Alder Reactions of (S)-2-(p-Tolylsulfinyl)- 1,4-benzoquinone.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
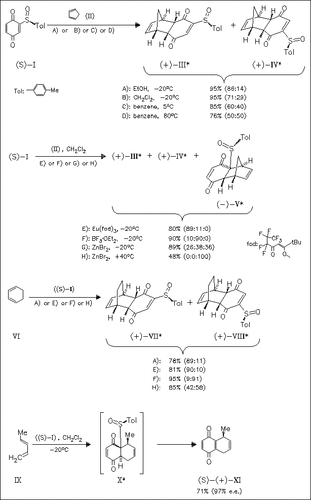
The title quinone (I) is found to react with cyclic dienes under thermal and Lewis acid conditions to afford cycloadducts resulting from attack at the unsubstituted C5-C6 double bond, with one exception. Reaction of (I) with cyclopentadiene in the presence of ZnBr2 also gives an adduct resulting from attack at the C2-C3 double bond. At 40 . degree.C it is the main product. The π-facial selectivity is affected by temperature, solvent, and present acid. In contrast, cycloaddition with acyclic dienes gives only products resulting from reaction on the C2-C3 double bond. The initially formed adducts spontaneously undergo elimination of the sulfinyl group giving compounds of type (XI). The results indicate that the sulfinyl group is able to control chemoselectivity and π-facial selectivity of cycloadditions.