ChemInform Abstract: Probing the Specificity of the Serine Proteases Subtilisin Carlsberg and α-Chymotrypsin with Enantiomeric 1-Acetamido Boronic Acids. An Unexpected Reversal of the Normal “L”-Stereoselectivity Preference.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
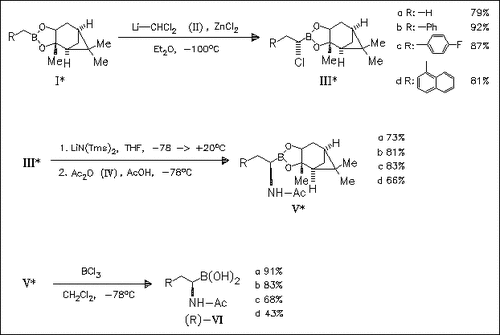
Both enantiomers (VI) are prepared as is demonstrated for the (R)- series starting with the homologation of the corresponding pinanediol esters. The α-chloroboronic compounds are obtained with diastereoselectivities >98%. All of the boronic acids are powerful competitive inhibitors of both title enzymes with the L-enantiomers being generally more potent than the D-enantiomers. However, a great reversal of the normal stereoselectivity preference is observed in the inhibition of α-chymotrypsin by (VId).