ChemInform Abstract: Synthesis and Photobiological Properties of 3-Acylangelicins, 3- Alkoxycarbonylangelicins and Related Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
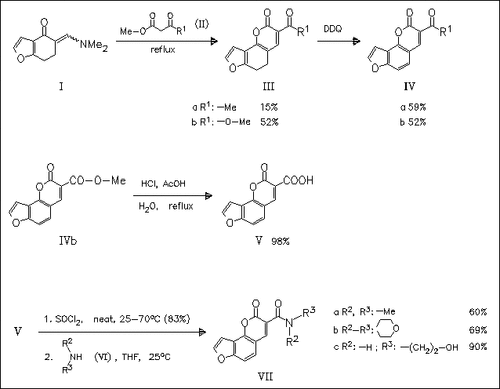
The synthesis of some new 3-acyl- and 3-alkoxycarbonylangelicins (cf. ( IVa) and (IVb), resp.) as well as related carboxamides (cf. (VII)) based on a 1,4-dipolar cycloaddition of β-ketoesters to α- aminomethyleneketone (I) and subsequent complete aromatization of the adducts with DDQ is accomplished. These new angelicin derivatives are assayed for their capacity to generate singlet oxygen under UVA irradiation, and their ability to induce antiproliferative effects. It is shown that these compounds represent a new model for non-toxic photochemotherapeutic drugs.