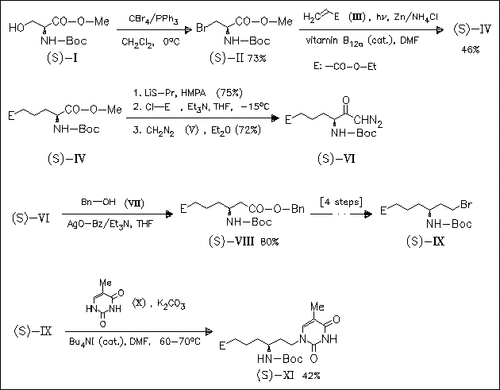
ChemInform Abstract: Synthesis of a Building Block for a Nucleic-Acid Analogue with a Chiral, Flexible Peptide Backbone.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The enantiomerically pure precursor (XI) of chiral and flexible nucleic acid analogues is synthesized in 9 steps starting from the L- serine derivative (I). Key steps are the vitamin B12a-catalyzed addition of (S)-(II) to (III) and the homologation of the free acid of (IV) into (VIII) by the Arndt-Eistert method.