ChemInform Abstract: Potent Human Immunodeficiency Virus Type 1 Protease Inhibitors That Utilize Noncoded D-Amino Acids as P2/P3 Ligands.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
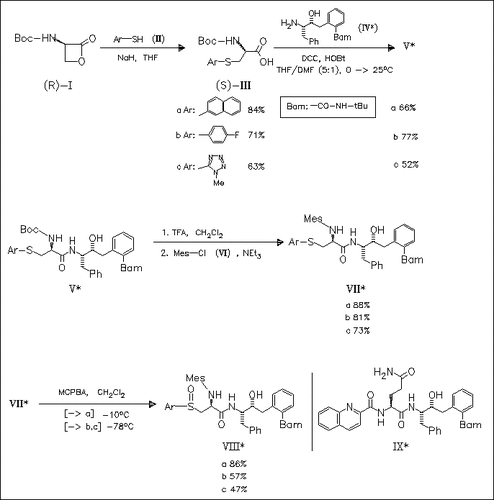
Noncoded D-amino acids are designed, which effectively replace the quinaldic amide-asparaginyl moiety (P2/P3 ligand) of the lead HIV-1 protease inhibitor LY289612 (IX) as evidenced by improved enzyme inhibitory and whole cell antiviral activity. Among the 36 compounds prepared, the derivatives (VIII) are found to be orally bioavailable in a rat model. All the amino acids are easily accessible starting with the ring opening reaction of the protected D-serine lactone (I).