ChemInform Abstract: Hetero-1,3-Dipolar Cycloadditions of Dithiolane-Isocyanate Imminium Methylides: A Novel Route to 1,3-Oxazolidine- and Thiazolidine-2- thiones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
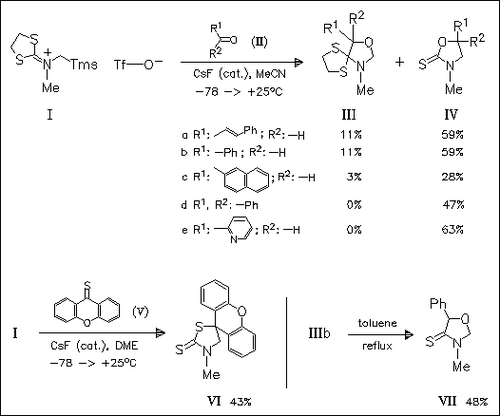
Treatment of the imminium salt (I) with CsF generates a dithiolane- isocyanate imminium methylide which is a new type of the azomethine methylide 1,3-dipole. It undergoes efficient and regioselective 1,3- dipolar cycloaddition with heterodienophiles (II) and (V) to form predominantly thiones (IV) and (VI) from the initial adducts via loss of thiirane.